Can a Baby Survive a Partial Molar Pregnancy
- Case report
- Open Access
- Published:
A partial tooth pregnancy associated with a fetus with intrauterine growth restriction delivered at 31 weeks: a instance study
Journal of Medical Case Reports volume 13, Article number:204 (2019) Cite this article
Abstract
Introduction
Tooth pregnancies vest to a group of diseases classified as gestational trophoblastic diseases, which result from an altered fertilization. Fractional molar pregnancy with a alive fetus is a very rare condition, occurring in 0.005 to 0.01% of all pregnancies; information technology presents a challenging diagnosis, specially when clinical signs are near completely absent.
Case presentation
Here we report a rare instance of partial molar pregnancy in which a normal-appearing male person fetus with diploid karyotype was delivered at 31 weeks gestation by a 37-year-old white woman. The pregnancy was characterized by an episode of threatened abortion in the first trimester and an ultrasonographic diagnosis of intrauterine growth restriction. Our patient did not report whatever suspicious symptoms for trophoblastic disease. Due to impaired umbilical avenue velocimetry with an absence of the diastolic stage, she underwent an emergency caesarean section at 31 weeks and delivered an 880 thou male person baby. The male baby was normal without any complications at iii-month and 12-month follow-up and the female parent had no prove of recurrence after 3 and 12 months of follow-up. Pathological examination of the placenta showed changes of partial hydatidiform mole.
Conclusion
Partial tooth pregnancy with a alive fetus is a very rare condition that presents a challenging diagnosis. Recognizing information technology is of primary importance for patient care and the placenta should always exist investigated at nativity, especially in a newborn with intrauterine growth brake.
Introduction
Hydatidiform mole belongs to a group of diseases classified every bit gestational trophoblastic disease, which results from an dumb fertilization [1, 2]. Moles have been divided into complete or partial hydatidiform moles on the basis of distinctive histopathological features and genetic abnormalities [3]. Partial molar pregnancy with circumstantial fetus is a rare complication with an incidence of 0.005–0.01% of all the pregnancies [ane]. Information technology usually derives from dispermic fertilization of a haploid normal oocyte and produces a triploid ready of chromosomes (69 Thirty, 69 XXY, or 69 XYY) and is most unremarkably associated with the presence of a malformed fetus [one, 2]. The most relevant symptom of a molar pregnancy is heavy bleeding from the vagina early in the pregnancy. Other symptoms tin can exist astringent nausea, hyperemesis, hyperthyroidism, hypertension, and proteinuria, and the occurrence of fetal anemia. Human being chorionic gonadotropin (hCG) levels are generally lower than in complete molar pregnancy [iii, 4]. A diagnosis can be accomplished with ultrasonography and sensitive measurement of serum hCG, usually on first trimester. However, partial moles are often misdiagnosed every bit an incomplete or missed abortion of the kickoff trimester. In less than 25% of cases, pregnancy with a fractional mole and a single normal fetus evolves to a viable fetus: in such cases the diagnosis can be suspected or misunderstood [five] and the management is a challenge for potential maternal and fetal complications. Hither we report a rare case of misdiagnosed fractional molar pregnancy in which a normal-appearing male person fetus with diploid karyotype was delivered at 31 weeks of gestation.
Example presentation
Our patient was informed of all procedures she was to undergo; she signed a consent allowing data drove for inquiry purposes, and gave total approval for the written report and publishing of the case. This example is in accordance with the Announcement of Helsinki, in accordance with the Consensus-based Clinical Case Reporting Guideline Evolution (http://www.equator-network.org/), and the Committee on Publication Ideals (COPE) guidelines (http://publicationethics.org/), and approved by the Institutional Review Board (IRB) of the university infirmary in which it was reported [6].
A 37-year-old white woman, gravida 1, at 30 weeks and 5 days of gestation was admitted in March 2018 to the Department of Adult female, Kid and Full general and Specialized Surgery of University of Campania "Luigi Vanvitelli" (Naples, Italy) with suspected fetal restricted growth. She had already attended the out-patient fertility center of our Department for a path of infertility, with the execution of genetic, laboratory, and antibiotic tests that gave a negative outcome. Subsequently, a spontaneous pregnancy occurred. Her obstetric history was characterized by an episode of threatened abortion in the showtime trimester. Serum titers of b-hCG were 7276 mIU/mL and 14,898 mIU/mL at 5 weeks and one twenty-four hour period and v weeks and 3 days of gestation, respectively. An ultrasound test at five weeks and 5 days revealed an empty gestational sac with no findings suspect for gestational trophoblastic disease. The following ultrasound examinations showed a regular ongoing pregnancy with a unmarried fetus. A structural ultrasound test at 20 weeks showed an altered menstruum charge per unit of uterine artery which justified the use of daily low-dose aspirin (100 mg) for prophylaxis of preeclampsia. Thyroid-stimulating hormone (TSH) levels in the first and 2nd trimester were in the normal range. A fetal echocardiography was performed at 24 weeks because not-invasive first-trimester screening for chromosomal abnormalities was non achieved: the effect was normal. Our patient was admitted to our department due to worsening of the biometric and Doppler velocimetry parameters, at thirty weeks and five days of gestation. An admission ultrasound exam revealed a singleton pregnancy with no fetal structural abnormalities, but fetal biometry was not consequent with gestation with an estimated weight of k g, below the third percentile for gestational age (consistent with 27 weeks). Therefore, a symmetrical intrauterine growth restriction (IUGR) was diagnosed. Amniotic fluid was regular in quantity and the placenta appeared regular. Umbilical artery Doppler velocimetry was impaired due to the absenteeism of the diastolic phase. Our patient was given ii injections of 12 mg of betamethasone 24 hours apart to prevent respiratory distress syndrome and she underwent an emergency cesarean section at 31 weeks and 1 twenty-four hours. A phenotypically normal alive and healthy male infant weighing 880 g was delivered. Macroscopic evaluation of the placenta showed a 14 × 10 × 3.5 cm discoid placenta with a weight of 416 g. On the maternal surface of the placenta, the presence of focal "grape" vesicles were observed, which occupied approximately five% of the peripheral surface (Fig. 1).
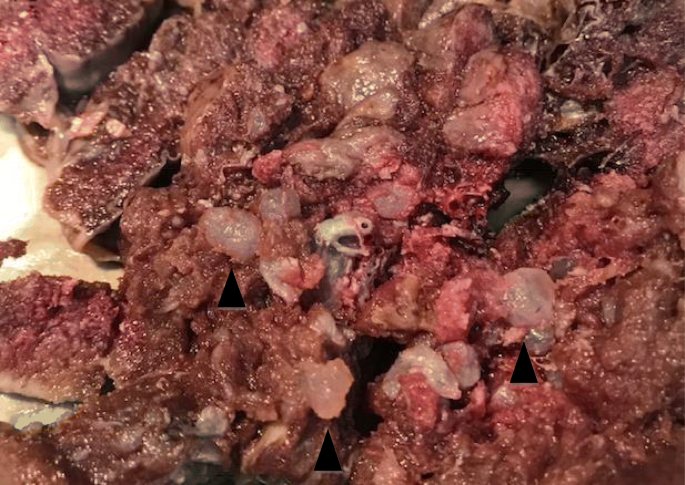
Hydatidiform molar villi. Annotation the bulbous swelling of terminal villi and the slender nature of main stem villi (black arrowhead)
On microscopic exam, these "grape-like areas" showed big-sized and medium-sized chorionic villi, with a festooned design, and numerous gross nodular swellings culminating in a "cistern-like" formation in the stromal axis and variable proliferation of trophoblast (Fig. 2) with normal claret vessels (Fig. iii).
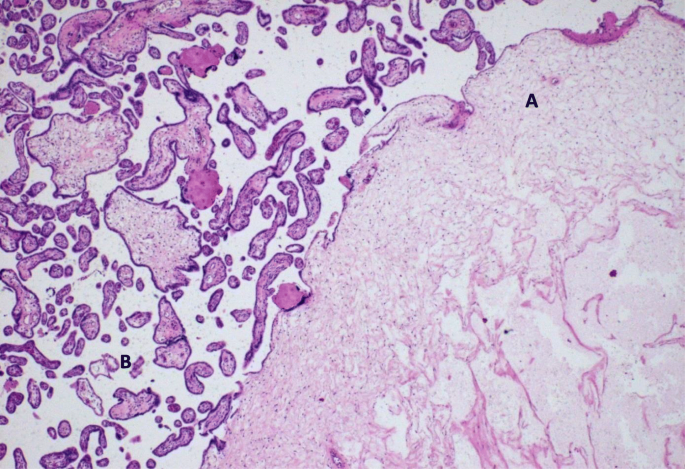
Focal intermingling of partial molar (A) with normal villi (B). Hematoxylin and eosin, × 400
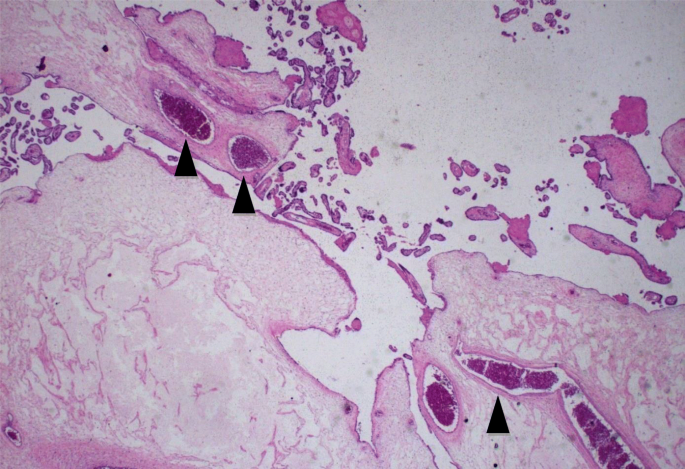
In this microscopic image is observed fractional molar villus with intact perfused fetal blood vessel (black arrowheads). Hematoxylin and eosin, × 400
The other 95% of the placenta was composed of pocket-sized, hypercapillarized chorionic villi (terminal villi). The pathological diagnosis was placenta at tertiary trimester with associated partial mole modifications. Our patient was in skilful health and had no evidence of recurrence later 3 and 12 months of follow-upward. The male baby was normal and the result of a karyotype assay was diploid. No complications occurred at 3-calendar month and 6-month follow-upward and the babe is nonetheless living. After delivery, hCG level was negative (less than 5 mIU/mL) and the mother had 12 sequent months of negative hCG levels and no metastases were found.
Discussion
Fractional molar pregnancy with circumstantial fetus is an extremely rare variation of a molar pregnancy: information technology accounts for 0.005 to 0.01% of all pregnancies and usually derives from dispermic fertilization of a haploid normal oocyte and produces a triploid set of chromosomes [i, 7]. An increased incidence could be explained by the greater utilize of assistive reproductive techniques [8]. There are three types of molar pregnancy with circumstantial normal alive fetus: the nearly frequent case is a twin pregnancy with i normal fetus having a normal placenta and another consummate mole; the second type is a twin pregnancy with regular fetus and placenta and another partial mole [ix]; the third and almost uncommon occurrence reported only nineteen times in the literature is a singleton normal fetus with partial tooth placenta, which is similar to our case [5]. The diagnosis of molar pregnancy with circumstantial fetus is difficult. In fact, the diagnosis tin be achieved with ultrasonography and sensitive measurement of serum hCG, usually on first trimester [x]. Moreover, in tooth pregnancy, symptoms are hyperemesis, heavy haemorrhage from the vagina, increased blood pressure and, sometimes, proteinuria that can lead to preeclampsia [seven, 10, 11]. Sometimes women with hydatidiform mole may feel symptoms typical of hyperthyroidism because of extremely high levels of hCG; in fact, this hormone tin mimic the action of TSH [12, 13]. High concentrations of hCG and suppressed levels of TSH tin aid to confirm the diagnosis. Interruption of pregnancy is common owing to built anomalies like triploidy of the fetuses and severe intrauterine fetal growth brake due to limited normal functional placental circulation. If the pregnancy does non terminate, management of tooth pregnancies with an obviously normal fetus remains challenging [5, xiv]. The woman must exist counseled regarding the maternal and fetal complications: late abortion, vaginal bleeding, mal presentations, preterm labor, persistent gestational trophoblastic disease, severe anemia in the fetus, hyperthyroidism, hypertensive disorders of pregnancy, pulmonary edema, and thromboembolic phenomena [15, 16]. Therefore, pregnancy needs to be followed with regular ultrasound cess of fetal anatomy and growth. A chorionic villous biopsy could be done if a live fetus is nowadays, to confirm the diagnosis and to differentiate between a partial mole and complete mole: it has been reported that the latter has approximately 20% tendency to become an invasive mole or even a choriocarcinoma, while the hazard was lower for fractional moles [fifteen, 17].
Determination
In our example, the diagnosis was not suspected and only the anomalous presence of IUGR could exist interpreted every bit a sign of a placental defect and, among the various causes, could be referred to as a molar pregnancy. As a consequence, histological examination of the placenta is essential in all cases of IUGR. Later on the delivery, it is mandatory to check the patient until there take been 12 sequent months of negative hCG levels. In conclusion, in every example of molar pregnancy suspect for a normal fetus, acceptable counseling of the patient and a strict follow-up during and after pregnancy for the chance of persistence of the trophoblastic pathology are necessary [eighteen,19,20].
Availability of data and materials
The dataset used and/or analyzed during the current report is available from the corresponding writer on reasonable request.
References
-
Smith HO, Kohorn East, Cole LA. Choriocarcinoma and gestational trophoblastic disease. Obstet Gynecol Clin North Am. 2005;32(4):661–84.
-
Soper JT. Gestational trophoblastic disease. Obstet Gynecol. 2006;108(i):176–87.
-
Stevens FT, Katzorke Northward, Tempfer C, Kreimer U, Bizjak GI, Fleisch MC, Fehm TN. Gestational Trophoblastic Disorders: An Update in 2015. Geburtshilfe Frauenheilkd. 2015;75(ten):1043–50.
-
Campitiello MR, De Franciscis P, Mele D, Izzo G, Sinisi A, Delrio M, Colacurci Due north. Endometrial LGR7 expression during menstrual wheel. Fertil Steril. 2011;95(8):2511–4.
-
Kawasaki Thou, Kondoh Due east, Minamiguchi Southward, Matsuda F, Higasa K, Fujita G, Mogami H, Chigusa Y, Konishi I. Live-born diploid foetus complicated with partial molar pregnancy presenting with pre-eclampsia, maternal anemia, and seemingly huge placenta: A rare case of confined placental mosaicism and literature review. J Obstet Gynaecol Res. 2016;42(8):911–7.
-
Gagnier JJ, Kienle 1000, Altman DG, Moher D, Sox H, Riley D, the CARE Group. The Intendance Guidelines: Consensus-based Clinical Case Reporting Guideline Development. BMJ Example Rep. 2013.
-
Hancock BW, Tidy JA. Electric current management of tooth pregnancy. J Reprod Med. 2002;47(5):347–54.
-
Malhotra N, Deka D, Takkar D, Kochar S, Goel S, Sharma MC. Hydatidiform mole with coexisting live foetus in dichorionic twin gestation. Eur J Obstet Gynecol Reprod Biol. 2001;94:301–3.
-
Hsieh CC, Hsieh TT, Hsueh C, Kuo DM, Lo LM, Hung TM. Commitment of a severely anaemic foetus after partial molar pregnancy; clinical and ultrasonographic finding. Hum Reported. 1999;14:1122–6.
-
Ross JA, Unipan A, Clarke J, Magee C, Johns J. Ultrasound diagnosis of tooth pregnancy. Ultrasound. 2018;26(3):153–nine.
-
Santos A, Trocado V, Gama AP, Pinheiro P, Nogueira R. Fractional Molar Pregnancy with Alive Foetus Diagnosed on 2nd Trimester: A Instance Report. J Gynecol Neonatal Biol. 2017;iii(two):1–four.
-
Wang Y, Qian H, Wang J. Medical termination of a partial hydatidiform mole and coexisting foetus during the second trimester: A instance report. Oncol Lett. 2015;ten(6):3625–8.
-
Walkington 50, Webster J, Hancock BW, Everard J, Coleman RE. Hyperthyroidism and human chorionic gonadotrophin production in gestational trophoblastic disease. Br J Cancer. 2011;104(eleven):1665–nine.
-
Siciliano RA, Mazzeo MF, Spada Five, Facchiano A, d'Acierno A, Stocchero 1000, De Franciscis P, Colacurci N, Sannolo N, Miraglia Due north. Rapid peptidomic profiling of peritoneal fluid by MALDI-TOF mass spectrometry for the identification of biomarkers of endometriosis. Gynecol Endocrinol. 2014;thirty(12):872–half dozen.
-
Rathod Due south, Samal SK, Ghose S. Twin pregnancy with hydatidiform mole and co-existing foetus: a instance study and review of literature. Int J Wellness Sci Res. 2014;4(seven):275–nine.
-
Simonelli A, Guadagni R, De Franciscis P, Colacurci Due north, Pieri Yard, Basilicata P, Pedata P, Lamberti M, Sannolo N, Miraglia North. Environmental and occupational exposure to bisphenol A and endometriosis: urinary and peritoneal fluid concentration levels. Int Arch Occup Environ Health. 2017;90(1):49–61.
-
Callen PW. Ultrasound evaluation of gestational trophoblastic disease. In: Callen Prisoner of war, editor. Ultrasonography in Obstetrics and Gynecology. 2nd ed. Philadelphia: W.B. Saunders; 1988. p. 416.
-
Worley MJ Jr, Joseph NT, Berkowitz RS, Goldstein DP. Women with a fractional mole during their first pregnancy and diagnosed earlier in gestation are at increased risk of developing gestational trophoblastic neoplasia. Int J Gynecol Cancer. 2014;24:941–five.
-
Berkowitz RS, Goldstein DP. Electric current advances in the management of gestational trophoblastic disease. Gynecol Oncol. 2013;128(i):3–5.
-
Khanom R, Khatun One thousand, Akter S. Pregnancy with a normal live foetus and a partial molar placenta - an extremely rare condition. J Dhaka Med Coll. 2009;18(i):82–4.
Acknowledgements
Not applicable.
Author information
Affiliations
Contributions
PDF and As designed and coordinated the work, nerveless the data, and corrected the manuscript. CT interpreted the patient data regarding the anamnesis. DL wrote the bibliography. EMM supervised the translation of the manuscript. ELM and MM wrote histological data. MT supervised the discussion and the conclusions. All authors read and canonical the final manuscript.
Corresponding author
Ethics declarations
Ethics approving and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case study and any accompanying images. A copy of the written consent is available for review by the Editor-in-Principal of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher'southward Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in whatsoever medium, provided you requite appropriate credit to the original writer(s) and the source, provide a link to the Creative Eatables license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/goose egg/1.0/) applies to the data made available in this commodity, unless otherwise stated.
Reprints and Permissions
About this commodity
Cite this commodity
De Franciscis, P., Schiattarella, A., Labriola, D. et al. A partial molar pregnancy associated with a fetus with intrauterine growth brake delivered at 31 weeks: a case written report. J Med Case Reports 13, 204 (2019). https://doi.org/x.1186/s13256-019-2150-4
-
Received:
-
Accustomed:
-
Published:
-
DOI : https://doi.org/10.1186/s13256-019-2150-4
Keywords
- IUGR
- Fractional molar pregnancy
- Gestational trophoblastic affliction
- Placental histologic exam
- Misdiagnosis
Source: https://jmedicalcasereports.biomedcentral.com/articles/10.1186/s13256-019-2150-4
Post a Comment for "Can a Baby Survive a Partial Molar Pregnancy"